Read time: 3 minutes.
This is Part 1 in a 3-part series explaining how new drugs and treatments get approved to treat lung cancer. Parts 2 and 3 will be published in the coming weeks.
Have you ever wondered how a new medicine or drug to treat lung cancer is brought to the people who need it? That’s what clinical trials help us do.
According to the National Cancer Institute, a clinical trial is a type of research study that tests how well new medical approaches (such as screening tests, prevention habits, or disease treatments) work in people. Sometimes, a clinical trial is called a clinical study.
Drug development often starts in a laboratory where chemical compounds (these are not drugs yet) are tested to see if they can kill cancer cells in a petri dish. When a compound passes this first test, it then moves forward and is checked to see if it can control the growth of cancer in mouse models and sometimes other animal models. Only a compound that passes these “pre-clinical” tests moves forward into a clinical trial.
There are four main phases of clinical trials. Each phase has its own purpose and goals. If those goals are met, the drug may move on to the next phase of testing.
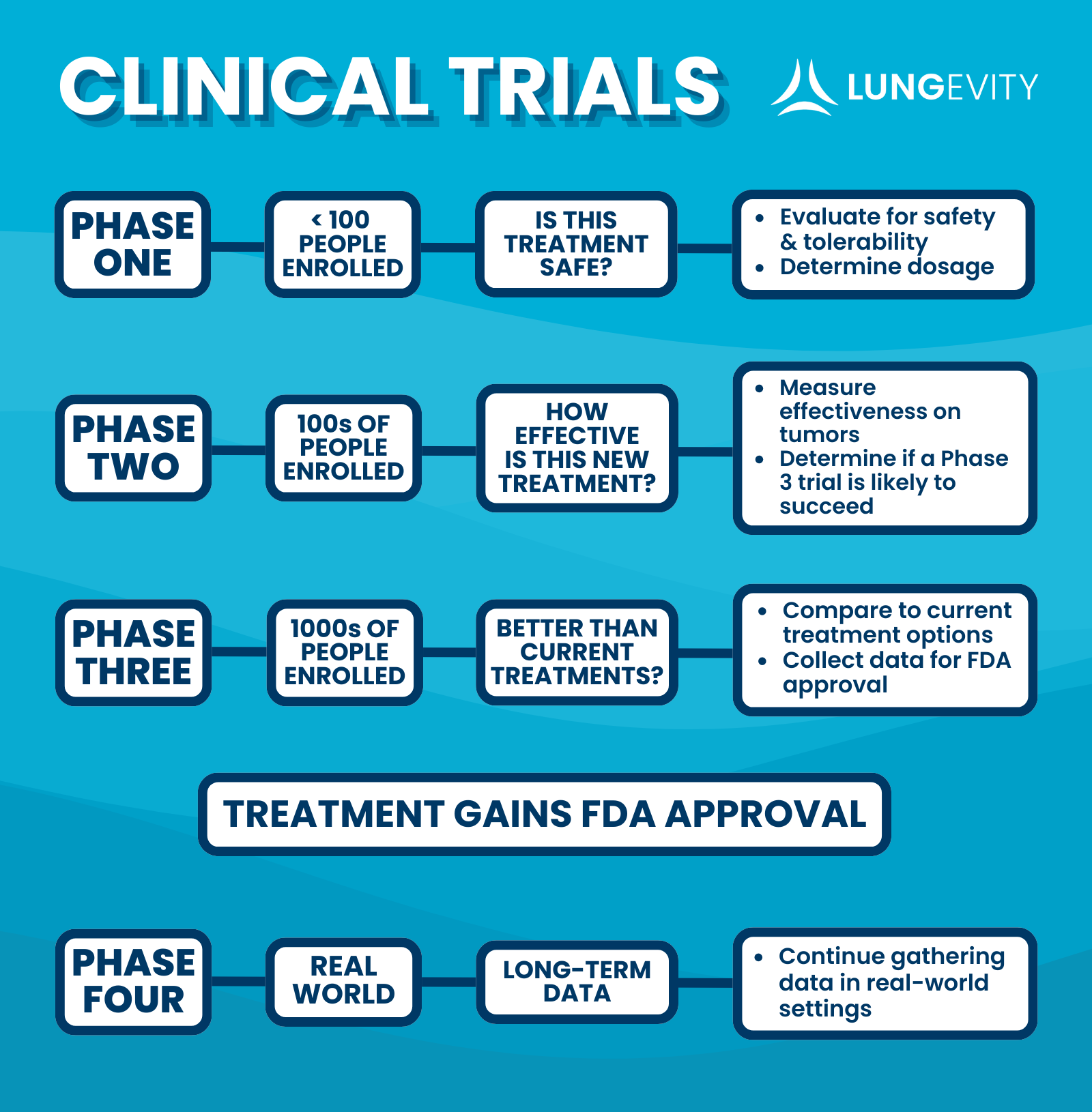
Phase 1: Is This Treatment Safe?
Once a treatment has enough laboratory data to back it up, researchers will test the treatment in humans through a phase 1 clinical trial. This trial is considered successful if researchers can show the treatment is safe, identify side effects and determine what the appropriate dosage would be. Phase 1 clinical trials are small—often enrolling fewer than 30 people in the trial. Sometimes Phase 1 trials are referred to as first in-human trials because a treatment is being tested for the first time in a person.
Phase 2: How Effective is This New Treatment?
Once the drug has been deemed safe for humans, the researchers need to demonstrate that the treatment is effective at shrinking the tumor. They will also continue learning about side effects and toxicities (the extent to which medications or treatments are harmful or poisonous). The phase 2 clinical trial lays the groundwork for the larger phase 3 clinical trials.
Phase 3: Is This Better Than Current Treatments?
When a drug enters a phase 3 clinical trial, there is often a lot of hope and excitement surrounding the treatment. It has been proven to be safe and effective in treating the disease in a small population. The phase 3 clinical trial is a large, expensive clinical trial. The treatment is typically compared to the current treatments available. Data from a Phase 3 clinical trial is submitted to the FDA (U.S. Food and Drug Administration) for review and could be the basis of a drug being approved for patient use in the United States.
Phase 4: Collecting Long-Term Real-World Data
Once the treatment gets FDA approval, researchers continue to monitor the effects of treatment. This data is often referred to as post-marketing data. Phase 4 clinical trial results look at the outcomes of the treatment in the real world over long periods of time—sometimes even decades. Through these trials, we continue to gather information, and sometimes we can learn things we did not know from previous trials. Many of the lung cancer treatments used today have gone through (or are currently going through) post-marketing studies.
The next question is—How do researchers decide if a clinical trial is successful? This will be answered in our next blog on clinical trial endpoints. Stay tuned!
If you like this type of information and want more of it, make sure to sign up for our email list.

