The goal of clinical trials is to find out whether new medical approaches that are being developed are safe, effective, and better than those currently being used. These approaches include screening, prevention, diagnosis, and treatment.1 Most drugs or medical procedures that are available for patients today went through clinical trial testing.

To help you understand and share this information, you can request our free booklet that summarizes the detailed information in the following sections.
While this section provides information about clinical trials in general, its focus is on trials that test new treatment options. Clinical trials are an important option for patients thinking about lung cancer treatments because the newest treatment approaches, not available otherwise, are being tested in them. Clinical trial research into lung cancer treatment options is possible only if patients with lung cancer volunteer to participate.
There are many ongoing clinical trials testing new lung cancer treatments, including targeted therapies, chemotherapy, radiation therapy, and immunotherapy, alone and in combination.
Finding a clinical trial that might be right for you
If you are considering participating in a clinical trial, start by asking your doctor whether there is one that might be a good match for you in your geographic area.
In addition, here are some resources to help you find one that may be a good match:
LUNGevity partners with Carebox, a clinical trials matching service, to help you with the decision of whether to participate in a clinical trial. Carebox helps you identify which lung cancer clinical trials you may be eligible for. The clinical trial navigators can also guide you through the process of getting enrolled if you choose to take part in a clinical trial. Clinical trial navigators are available Monday through Friday from 9 am to 6 pm ET at 877-769-4834 (in both English and Spanish). Learn more about this free service and fill out an online profile to help identify clinical trials that might be a good match for you.
Information about available clinical trials is also available through these websites:
- National Cancer Institute clinical trials search: This site includes all of the thousands of clinical trials in the United States in all cancer types.
- My Cancer Genome: This resource is managed by a team of doctors at Vanderbilt University. My Cancer Genome gives up-to-date information on what mutations make cancers grow and related treatment options, including available clinical trials.
- Lung Cancer Master Protocol (Lung-MAP): For patients with advanced non-small cell lung cancer, Lung-MAP is a collaboration of many research sites across the country, using a unique approach to matching patients to one of several drugs being developed.
In addition, if you are interested in a specific drug or other treatment that is being developed, you can often find information about studies for that drug on the website of the company developing it.
What is a clinical trial?
Clinical trials are research studies that take place in a medical, or clinical, setting. These studies test new medical approaches for their safety and efficacyThe ability of an intervention (for example, a drug or surgery) to produce the desired beneficial effect among a group of volunteer participants.
Clinical trials are led by a principal investigator (PI), who is frequently a medical doctor. The PI is supported by a research team. Members of the research team include a number of healthcare professionals, among them doctors, nurses, and social workers. Members of the healthcare team monitor a participant's health regularly while they are enrolled in the clinical trial.2
For patients with lung cancer or at high risk for it, clinical trials test new ways to:3
- Find and diagnose lung cancer
- Treat lung cancer
- Manage symptoms of the lung cancer or side effects of treatment (both short-term and long-term)
- Prevent lung cancer in the first place (through chemopreventionThe use of drugs, vitamins, or other agents to try to reduce the risk of, or delay the development or recurrence of, cancer, for example)
Clinical trials are vital for adding to medical knowledge that can improve the care given to patients. The results of clinical trials determine whether new treatments are approved by the FDA for prescribing by doctors for patients outside clinical trials.
Before a treatment is studied in humans in a clinical trial, it has been studied in a pre-clinical research phase. During that time, the treatment is generally studied in laboratory animals for safety and efficacy. Treatments do not move into clinical trials unless there is reason from pre-clinical research results to believe that the treatment will provide a benefit to humans.4
What is a clinical trial protocol?
Each clinical trial has its own study plan, or protocolA detailed plan of a scientific or medical experiment, treatment, or procedure. The protocol outlines every aspect of how the clinical trial is to be conducted. For a clinical trial testing a new treatment, the protocol includes:2,5,6
- The reason for doing the clinical trial: its aims
- Who can participate in the clinical trial, and how many
- How long the clinical trial will last
- What treatment is given, how the treatment is given, and how often the treatment is given
- What medical tests will be done to measure whether the treatment is working
- What types of information will be collected about the participants
- What safeguards there will be for participants
Patients who participate in treatment-focused clinical trials receive either a new treatment/combination of treatments or the treatment that is currently considered the standard of care, which is the best currently available proven treatment. A patient participating in a clinical trial will never be given less than the current standard of care.
Clinical trials vary in how long they last. Participants are told the length before they sign up.2
Who can participate in a clinical trial?
Each clinical trial defines who is eligible to take part. Each clinical trial includes only those patients who have the required traits, known as the eligibility criteria. The critera that allow someone to participate in a clinical trial are called inclusion criteria, while the factors that disqualify someone from participating are called exclusion criteria. Criteria for who may take part in a clinical trial might include:2,8,9,10
- A specific age range
- Gender
- The type of lung cancer—could be by histologyThe study of tissues and cells under a microscope and/or genomic mutationAny change in the gene sequence of a cell
- The stage of lung cancer
- Site of metastasesThe spread of cancer from the primary site, or place where it started, to other places in the body—for example, whether someone has brain (or central nervous system) metastases
- Previous treatment history—type of treatment or how many different types of treatment
- Medical history
- Current health status
Criteria such as these help reduce the medical differences among participants in the clinical trial. When those taking part in a clinical trial are alike in key ways, researchers can be more certain that the results are due to the treatment being tested and not to other factors.8,9
Also, some patients have health problems in addition to lung cancer that could be made worse by the treatments in a clinical trial. If you are interested in participating in a clinical trial, you will receive medical tests to be sure that you are medically fit for it.8,9
Where do clinical trials take place?
There is no one standard for where clinical trials take place. Some are available in just a few places; others have sites at many locations across the United States and in numerous other countries. They take place in locations such as doctors’ offices, community hospitals and clinics, medical centers, cancer centers (including NCI-designated cancer centers), veterans’ and military hospitals, and the National Institutes of Health Clinical Center.2,11
When you speak with your doctors about treatment options, ask about clinical trials in your geographic area. In addition, there are other resources to help you find a clinical trial that might be right for you; these are outlined later in this section.
What are the phases of a clinical trial?
Clinical trials to test new cancer treatments involve several steps, called phases. There are typically three main phases of clinical trials. If a new treatment is successful in one phase, it will move on to further testing in the next phase. A new treatment usually must show success in the first three phases before it is approved by the US Food and Drug Administration (FDA) for broader use.
However, the FDA also has a number of programs to support faster development and review of new drugs in areas of unmet medical need, which can include lung cancer. These programs include accelerated approval. A treatment may be given accelerated approval by the FDA after strong phase 2 results, for example, and then monitored closely afterward.12
In some cases, a new treatment will undergo an additional phase, phase 4—this is also called post-marketing surveillance. The goal in phase 4 is to monitor in the real world the long-term effectiveness and side effects of an approved treatment after it is on the market with FDA approval.6
The following table, taken primarily from a National Cancer Institute (NCI) outline of clinical trial phases, shows the purpose of, and the approximate number of participants who take part in, each of the common phases of a cancer treatment clinical trial. A participant typically participates in only one phase of a clinical trial.4,13,14,15
|
Phase |
Goals |
Approximate Number of Participants |
|
Phase 1 |
|
|
|
Phase 2 |
|
|
|
Phase 3 |
|
|
|
Phase 4 |
|
|
Some PIs design clinical trials that combine two phases (phase 1/2 or phase 2/3 trials) in a single protocol. This combined design may allow research questions to be answered more quickly or with fewer patients.4
How are patients assigned to different treatments in clinical trials?
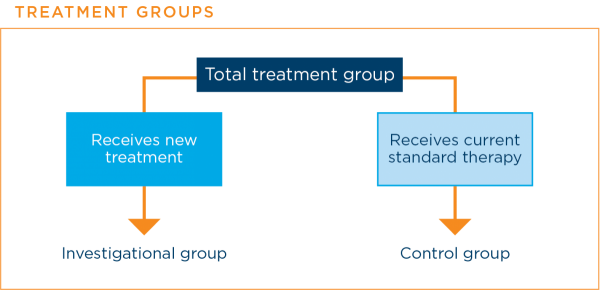
In some phase 2 clinical trials and all phase 3 clinical trials, there are at least two groups to which patients are assigned. Each of these groups receives a different treatment.
The assignments are made randomly, usually by a computer, so that neither the researchers nor the patient knows which group the patient is in. This means that the results are affected as little as possible by factors other than the treatment received by the patient.
A group that includes patients who receive the new treatment is called the investigational groupIn a treatment clinical trial, the group that receives the new treatment being studied. A group that includes patients who receive the current standard therapy is called the control groupIn a clinical trial, the group that does not receive the new treatment being studied.16
When are placebos used in clinical trials?
A placebo is an inactive substance with no medical properties. In lung cancer clinical trials, patients are never given a placebo instead of an effective standard treatment.
Placebos are sometimes used in cancer treatment clinical trials. They are used when a clinical trial is comparing a current standard treatment plus a new treatment with the current standard treatment alone. The placebo is designed to look like the new treatment, but it is not active. Using a placebo in this way prevents patients and their doctors from knowing to which treatment group a patient was assigned. This helps to prevent bias in the results.3,7,16
The graphic below shows how a placebo might be used in a clinical trial.
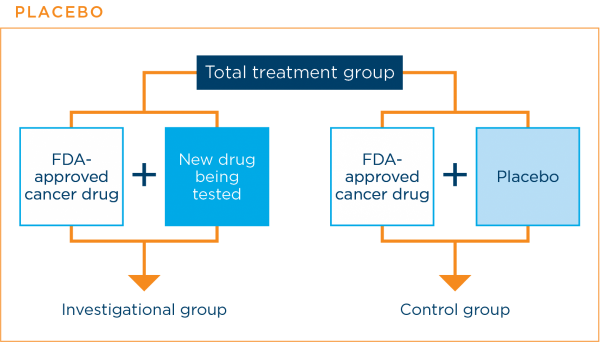
The research team will always let you know if the clinical trial will use a placebo along with a standard treatment.3,7,16
What happens after the clinical trial is over?
After a clinical trial is over, the PI and research team analyze the results to determine what the next steps should be, based on the efficacy and safety results. A clinical trial may be stopped or may move on to the next phase. A treatment with strong, positive phase 3 results may move on to approval by the FDA. Often, results of a clinical trial are published; you can check with the clinical trial research team to find out about this.10
The clinical trial’s research team may remain in contact with you and let you know about the trial’s findings and conclusions. They may ask you to continue to provide information about your health either through surveys or actual health examinations. This is in addition to the regular care provided by your own healthcare team.17
How does a clinical trial fit in with a patient’s overall health care?
The clinical trial protocol determines how often a patient receives a drug treatment and what exactly that treatment will be. Most often, patients taking part in a clinical trial will continue to receive the rest of their healthcare from their regular healthcare team. The researchers and regular healthcare team can work together to ensure that the clinical trial protocol will not conflict with any other drugs or treatments that the patient is taking.2
How are patients in clinical trials kept safe?
Patients who take part in clinical trials are protected in a number of ways. The federal government has specific rules that help ensure that clinical trials are conducted both safely and ethically.2,3,18,19
Ways to keep people safe include:
Informed consentA process in which patients are given important information, including possible risks and benefits, about a medical procedure or treatment, a clinical trial, or genetic testing to help them decide if they want to be treated, tested, or take part in the trial process: Researchers must provide patients who are thinking about participating in a clinical trial with detailed information about it. This includes enough information about the clinical trial’s purpose and possible risks and benefits to allow a patient to make the best decision about whether to take part. This information must be given in writing and may be discussed with the doctors and nurses on the research team. If a person decides to take part in a clinical trial, he or she will be given an informed consent document to sign, confirming understanding of this information.
This informed consent document is not a contract. Participants always have the right to leave the clinical trial at any time, even if it is not over yet.
Review of the clinical trial protocol by an institutional review board (IRB)A group of scientists, doctors, clergy, and patient advocates that reviews and approves the detailed plan for every clinical trial: There is an IRB at every healthcare facility that does clinical research. This board includes doctors, researchers, and community members. The IRB’s role is to ensure that the clinical trial protocol is ethical and that the rights and welfare of the participants who take part are being protected.
Ongoing monitoring of the clinical trial: Once the clinical trial is under way, it continues to be monitored by the IRB as well as by the sponsor of the trial, the research team conducting the trial, and, for the phase 3 portion of a clinical trial, a Data and Safety Monitoring Board (DSMB)An impartial group that oversees a clinical trial and reviews the results to see if they are acceptable, which decides whether a trial should be changed or closed. The DSMB is made up of doctors, statisticians, and others who are independent of the people, organizations, and institutions that are sponsoring, organizing, and conducting the clinical trial. DSMB members are experts in clinical research and clinical trials. The FDA also meets with researchers and inspects the clinical trial sites.
What are the benefits and risks associated with a clinical trial?
As with all treatment options, there are both benefits and risks for those who take part in a clinical trial. Before deciding to take part, you should carefully consider both. If you are thinking about taking part in a clinical trial, ask your healthcare team and the clinical trial research team as many questions as you need to until you are satisfied that you understand exactly what is involved. Here are lists from the National Cancer Institute (NCI) of possible benefits and possible risks of participation:8
Possible benefits:
- You will have access to a new treatment that is not otherwise available, reflecting the latest thinking in lung cancer research and treatment
- The research team will monitor your health closely, providing excellent care
- If the treatment being studied is more effective than the standard treatment, you may be among the first to benefit
- Even if you do not directly benefit, the information gathered during the clinical trial will still add to knowledge about lung cancer and can help other people
Possible risks:
- The new treatment may be no better than, or even as good as, the standard treatment
- New treatments may have side effects that doctors do not expect or that are worse than those of the standard treatment
- You may be required to make more visits to the doctor or have more hospitalizations than if you were receiving standard treatment
- You may need extra tests, and some of these may be uncomfortable or time-consuming
- Even if a new treatment has benefits in some patients, it may not benefit you
- Health insurance may not cover all patient care costs in a trial. In addition, you may have extra expenses related to extra doctor visits, such as travel and childcare costs
Paying for clinical trials
There are two types of costs that are associated with a clinical trial: patient care costs and research costs. Below are NCI lists of what is included in these costs.20
Patient care costs are costs related to treating your cancer, whether you are in a clinical trial or are receiving standard care. These costs are covered by most health insurance plans, but it is important for you to check to determine exactly what is covered.
Costs include:
- Doctor visits
- Hospital stays
- Lab tests
- X-rays and other imaging tests
- Standard cancer treatment
- Treatment for symptom and side-effect relief
Research costs are costs related to taking part in a clinical trial. These costs are often not covered by health insurance plans, but they may be covered by the clinical trial’s sponsor, the organization or entity funding the clinical trial. (Read further for a list of possible sponsors.) Again, it is important for you to check to determine exactly what is covered.
Costs include include:
- The treatment under study
- Lab tests performed solely for research purposes
- Additional X-rays and imaging tests performed solely for research purposes
Additionally, when you take part in a clinical trial, you may have extra doctor visits that you would not have with standard treatment. These extra visits can add costs for transportation and child care. Information about resources that can provide help with these and other costs can be found in Support & Survivorship.
Clinical trials may be sponsored by a number of different organizations and entities, including:2
- Pharmaceutical or biotechnology companies
- Academic medical centers
- Community hospitals
- Government agencies, such as the National Institutes of Health, the US Department of Defense, and the US Department of Veterans Affairs
- Physicians
- Patient advocacy groups
Updated March 16, 2021
References
- Clinical Trial. NCI Dictionary of Cancer Terms website. https://www.cancer.gov/publications/dictionaries/cancer-terms/. Accessed March 15, 2021.
- Learn about Clinical Studies. ClinicalTrials.gov website. https://clinicaltrials.gov/ct2/about-studies/learn. Accessed March 15, 2021.
- Lung Cancer - Non-Small Cell: About Clinical Trials. Cancer.Net website. https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/about-clinical-trials. Approved May 2020. Accessed March 15, 2021.
- Phases of Clinical Trials. National Cancer Institute website. https://www.cancer.gov/about-cancer/treatment/clinical-trials/what-are-trials/phases. Updated February 6, 2020. Accessed March 15, 2021.
- Clinical Trial Protocol Development. UCSF Clinical Research Resource HUB website. https://hub.ucsf.edu/protocol-development. Reviewed Octrober 3, 2017. Accessed March 15, 2021.
- Al-Jundi A , Sakka S. Protocol Writing in Clinical Research. J Clin Diagn Res. 2016 Nov; 10(11); ZE10-ZE13. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5198475/. Published online November 1, 2016. Accessed March 15, 2021.
- Use of Placebos. National Cancer Institute website. https://www.cancer.gov/about-cancer/treatment/clinical-trials/what-are-trials/placebo. Reviewed February 6, 2020. Accessed March 15, 2021.
- Deciding to Take Part in a Clinical Trial. National Cancer Institute website. https://www.cancer.gov/about-cancer/treatment/clinical-trials/taking-part. Reviewed Februrary 12, 2020. Accessed March 15, 2021.
- Deciding Whether to Be Part of a Clinical Trial. American Cancer Society website. https://www.cancer.org/treatment/treatments-and-side-effects/clinical-trials/what-you-need-to-know/who-does-clinical-trials.html. Revised August 18, 2020. Accessed March 15, 2021.
- NIH Clinical Research Trials and You: The Basics. National Institutes of Health (NIH) website. https://www.nih.gov/health-information/nih-clinical-research-trials-you/basics. Reviewed October 20, 2017. Accessed March 15, 2021.
- Where Trials Take Place. National Cancer Institute website. https://www.cancer.gov/about-cancer/treatment/clinical-trials/what-are-trials/where. Updated February 4, 2020. Accessed March 15, 2021.
- Fast Track, Breakthrough Therapy, Accelerated Approval, Priority Review. US Food and Drug Administration website. https://www.fda.gov/patients/learn-about-drug-and-device-approvals/fast-track-breakthrough-therapy-accelerated-approval-priority-review. Accessed March 15, 2021.
- Mandal A. What is a phase 4 trial? News Medical Life Sciences website. https://www.news-medical.net/health/What-is-a-Phase-4-Clinical-Trial.aspx. Updated February 26, 2019. Accessed March 15, 2021.
- Phases of clinical trials. National Comprehensive Cancer Network (NCCN) website. https://www.nccn.org/patients/resources/clinical_trials/phases.aspx. Copyright 2021. Accessed March 15, 2021.
- Phases of Clinical Trials. The University of Texas MD Anderson Cancer Center website. https://www.mdanderson.org/patients-family/diagnosis-treatment/clinical-trials/phases-of-clinical-trials.html. Copyright 2021. Accessed March 15, 2021.
- Randomization and Bias in Cancer Clinical Trials. National Cancer Institute website. https://www.cancer.gov/about-cancer/treatment/clinical-trials/what-are-trials/randomization. Reviewed February 6, 2020. Accessed March 15, 2021.
- Clinical Trials and Me: After my clinical trial. Abbvie website. https://www.clinicaltrialsandme.com/resources/after-my-clinical-trial.html#:~:text=If%20a%20clinical%20trial%20ends,for%20which%20you%20may%20qualify. Copyright 2021. Accessed March 15, 2021.
- Informed Consent. National Cancer Institute website. https://www.cancer.gov/about-cancer/treatment/clinical-trials/patient-safety/informed-consent. Reviewed February 11, 2020. Accessed March 16, 2021.
- Patient Safety in Clinical Trials. National Cancer Institute website. https://www.cancer.gov/about-cancer/treatment/clinical-trials/patient-safety. Reviewed February 6, 2020. Accessed March 16, 2021.
- Paying for Clinical Trials. National Cancer Institute website. https://www.cancer.gov/about-cancer/treatment/clinical-trials/paying. Updated February 6, 2020. Accessed March 16, 2021.

