The process by which the body makes new blood vessels is called angiogenesis. When new blood vessels transport oxygen and nutrients to the cancer cells of a tumor, they help the tumor to grow and spread.
The goal of drugs known as angiogenesis inhibitors is to help stop or slow the growth or spread of tumorsAn abnormal mass of tissue that results when cells divide more than they should or do not die when they should by stopping the formation of blood vessels that go to them. They do not work to stop the growth of the tumor cells themselves.1,2
How do angiogenesis inhibitors work?
Vascular endothelial growth factor (VEGF) is a protein released from the tumor cells that can attach to the VEGF receptors on blood vessel cells and help new blood vessels form around the tumors. The blood vessels, with their supply of oxygen and nutrients, can help the cancer cells grow.1,3
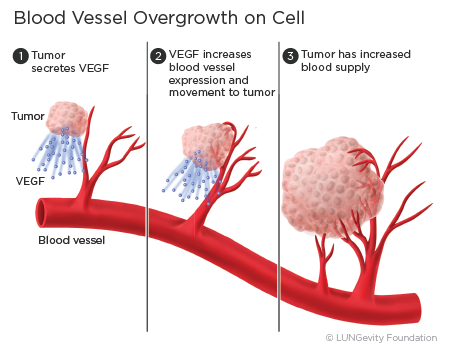
Angiogenesis inhibitors work by specifically recognizing and binding to VEGF so that VEGF is unable to activate the VEGF receptor to stimulate the growth of new blood vessels.
Because angiogenesis inhibitors do not kill tumors but instead prevent tumors from growing, they may need to be administered over a long period. In addition, angiogenesis inhibitors are most effective when used in combination with other therapies, and they are approved by the US Food and Drug Administration (FDA) only in those combinations.1,2,3
Approved angiogenesis inhibitors for non-small cell lung cancer (NSCLC)
Currently, two angiogenesis inhibitors are FDA-approved for treatment of NSCLC. There are no angiogenesis inhibitors approved for small cell lung cancer.
Note: Bevacizumab (Avastin®) is not approved or recommended for treating squamous cell NSCLCA type of non-small cell lung cancer that usually starts near a central bronchus. It has been found to cause serious bleeding from the lungs of squamous cell NSCLC patients.6
- Bevacizumab (Avastin®):4,5 Approved as:
- first-line treatmentThe first treatment given for a disease of unresectableUnable to be removed by surgery, locally advancedHaving spread from where it started to nearby tissue or lymph nodes, recurrentHaving come back after a period of time during which the cancer could not be detected, or metastaticHaving spread from the primary site, or place where it started non–squamous NSCLC in combination with the chemotherapiesTreatment with drugs that kill cancer cells carboplatin (Paraplatin®) and paclitaxel (Taxol® or Onxol®)
- first-line treatment for adult patients with metastatic non-squamous NSCLC with no EGFR or ALK mutations, in combination with paclitaxel (Taxol® or Onxol®), carboplatin (Paraplatin®), and the immunotherapy drug atezolizumab (Tecentriq®)
- Ramucirumab (Cyramza®):7 Approved:
- in combination with the chemotherapy docetaxel (Taxotere®) for the second- or subsequent-line treatmentTreatment that is usually started after the first set of treatments doesn’t work, has stopped working, or has side effects that are not tolerated of patients with metastaticHaving spread from the primary site, or place where it started non-small cell lung cancer whose disease has progressed while on or after taking platinum-based chemotherapy (for example, cisplatin (Platinol-AQ®) or carboplatin (Paraplatin®)). Patients with EGFR or ALK mutations should have disease progression on FDA-approved targeted therapyA type of treatment that uses drugs to attack specific types of cancer cells with less harm to normal cells for these mutations before receiving ramucirumab (Cyramza®)
- in combination with erlotinib (Tarceva®) for first-line treatment of patients with metastatic EGFR exon 19 deletion or exon 21 (L858R) substitution mutations
How Are They Administered?
Bevacizumab (Avastin®) is given intravenously:4,5
- when given in combination with carboplatin (Paraplatin®) and paclitaxel (Taxol® or Onxol®): 15 mg/kg every 3 weeks
- when given in combination with carboplatin (Paraplatin®) and paclitaxel (Taxol® or Onxol®) and atezolizumab (Tecentriq®): 15 mg/kg every 3 weeks. When given on the same day as atezolizumab (Tecentriq®), the atezolizumab (Tecentriq®) is administered earlier.
Ramucirumab (Cyramza®) is given intravenously:7
- when given with docataxel (Taxotere®): 10 mg/kg on Day 1 of a 21-day cycle prior to docetaxel infusion
- when given with erlotinib (Tarceva®): 10mg/kg every 2 weeks
What are the side effects of angiogenesis inhibitors?
Like any treatment, angiogenesis inhibitors can cause side effects. Each drug has a different set of most common side effects. It’s important to remember that just because a side effect is possible doesn’t mean that a patient will experience it. Before taking an angiogenesis inhibitor, which is approved only in combination with other therapies, a patient should discuss with their healthcare team what side effects they might expect from the treatments and how to prevent or ease them. A patient should speak with their healthcare team if and when new side effects begin, as treating them early on is often more effective than trying to treat them once they have already become severe.
Bevacizumab (avastin®)
Common side effects of bevacizumab (Avastin®) include nosebleeds, headaches, high blood pressure, inflammation of the nose, too much protein in the urine, taste alteration, dry skin, hemorrhage, increased tearing of the eyes, back pain, and redness and peeling of the skin.4
Rarer and more serious side effects may occur, including bleeding from the stomach and intestines, which can be severe. Other serious side effects may include clots in the lungs and legs, heart attacks, and strokes. It also interferes with the healing of wounds.4
Ramucirumab (cyramza®)
The most common side effects seen in patients treated with the combination of ramucirumab (Cyramza®) and docetaxel (Taxotere®) include neutropeniaA condition in which there are fewer than normal neutrophils (a type of white blood cell), leading to increased susceptibility to infection, fatigue/weakness, and stomatitisInflammation or irritation of the mucous membranes in the mouth and irritation or inflammation of other mucous membranes. Rarer side effects include febrile neutropeniaA condition marked by fever and a lower-than-normal number of neutrophils in the blood, swelling from fluid build-up in the hands and lower legs, thrombocytopeniaA condition in which there are fewer platelets in the blood than normal, increased blood pressure, increased tearing of the eyes, and nosebleeds.7
The most common side effects seen in patients treated with ramucirumab (Cyramza®) with erlotinib (Tarceva®) are infections, high blood pressure, stomatitis, excess protein in the urine, hair loss, and nosebleeds.6
Finding a clinical trial that might be right for you
Clinical research is ongoing to evaluate the role of angiogenesis inhibitors in a range of other lung cancer treatment approaches, including alone or in combination with other drugs.8
If you are considering participating in a clinical trial, start by asking your healthcare team whether there is one for which you might qualify in your area.
LUNGevity partners with Carebox, a clinical trials matching service, to help you with the decision of whether to participate in a clinical trial. Carebox helps you identify which lung cancer clinical trials you may be eligible for. The clinical trial navigators can also guide you through the process of getting enrolled if you choose to take part in a clinical trial. Clinical trial navigators are available Monday through Friday from 9:00 am to 5:00 pm ET at 877-769-4834 (in both English and Spanish). Learn more about this free service and even fill out an online profile to help identify clinical trials that might be a good match for you.
Information about available clinical trials is also available through these websites:
- National Cancer Institute clinical trials search: This site includes all of the thousands of clinical trials in the United States in all cancer types.
- My Cancer Genome: This resource is managed by a team of doctors at Vanderbilt University. My Cancer Genome gives up-to-date information on what mutations make cancers grow and related treatment options, including available clinical trials.
- Lung Cancer Master Protocol (Lung-MAP): For patients with advanced non-small cell lung cancer, Lung-MAP is a collaboration of many research sites across the country, using a unique approach to matching patients to one of several drugs being developed.
Updated March 9, 2021
References
- Angiogenesis Inhibitors. National Cancer Institute website. https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/angiogenesis-inhibitors-fact-sheet. Reviewed April 2, 2018. Accessed March 9, 2021.
- Angiogenesis and angiogenesis inhibitors to treat cancer. Cancer.Net website. https://www.cancer.net/navigating-cancer-care/how-cancer-treated/personalized-and-targeted-therapies/angiogenesis-and-angiogenesis-inhibitors-treat-c…. Approved November 2018. Accessed March 9, 2021.
- Angiogenesis Inhibitors. The University of Texas MD Anderson Cancer Center. https://www.mdanderson.org/treatment-options/angiogenesis-inhibitors.html. Copyright 2021. Accessed March 9, 2021.
- Avastin® (bevacizumab) [package insert]. Genentech, Inc. South San Francisco, CA. November 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125085s332lbl.pdf. Revised May 2020. Accessed March 9, 2021.
- Tecentriq® (atezolizumab) [package insert]. Genentech, Inc. South San Francisco, CA. 2016. https://www.gene.com/download/pd/tecentriq_prescribing.pdf. Revised May 2020. Accessed March 9, 2021.
- Avastin safety profile. Avastin website. https://www.avastin.com/hcp/safety.html. Copyright 2021. Accessed March 9, 2021.
- Cyramza® (ramucirumab) [package insert]. Eli Lilly and Company. Indianapolis, IN; December 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125477s034lbl.pdf. Revised May 2020. Accessed March 9, 2021.
- Clinicaltrials.gov. US National Institutes of Health website. http://clinicaltrials.gov. Accessed December March 9, 2021.
