Read time: 5 minutes.
This is Part 3 in our series on how drugs get approved to treat lung cancer.
The United States federal government aims to regulate prescription drugs to ensure people are receiving medication that’s safe and effective. Every prescribed drug in the U.S. has gone through a rigorous testing process that can take over a decade to complete before the U.S. Food and Drug Administration (FDA) approves the drug and people can benefit from treatment.
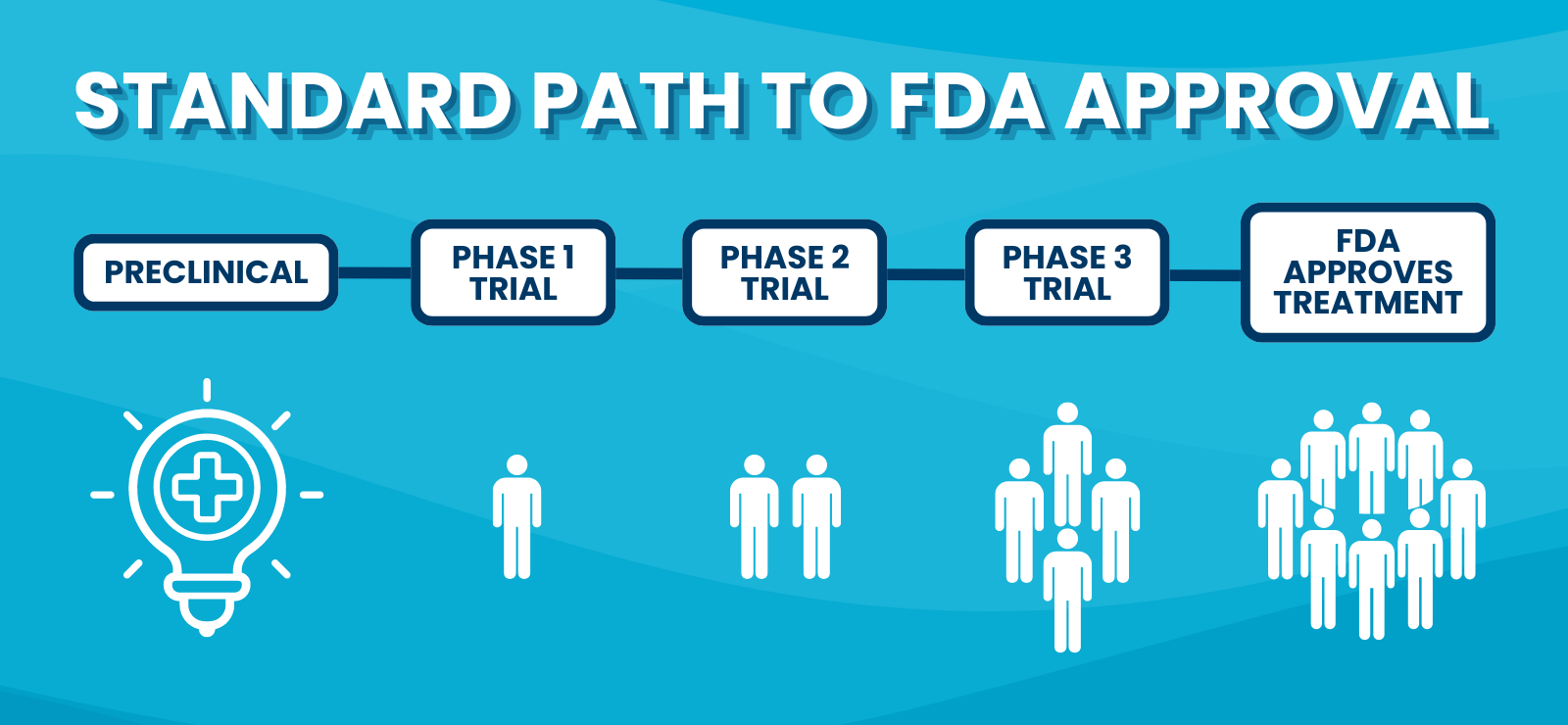
Because people often want access to treatments sooner, the FDA has developed ways to speed up the approval process for certain diseases—including cancer. There are now multiple paths for cancer drugs to be evaluated and (hopefully) approved by the FDA. Whichever path the researchers take, the drugs go through laboratory testing and clinical trials to demonstrate safety and effectiveness before the FDA approves them.
FDA Designations for Cancer Treatments
As drugs go through the approval process, they may receive a special status, or designation, to help them move through the process more quickly. The three most common FDA designations for cancer treatments are explained below.
- Priority Review Designation – In 1992, the FDA established a two-tiered system of review times—standard review and priority review. A priority review designation means the FDA’s goal is to make a decision within 6 months of receiving the application. This is 4 months earlier than the standard review time of 10 months.
- Breakthrough Therapy Designation – The FDA uses this designation to speed the development and review of the drugs that are intended to treat a serious condition. In these cases, researchers have preliminary data that indicates the drug is likely to be a substantial improvement over current treatment. This designation typically happens by the end of phase 2 clinical trials and sets up the drug to move quickly through the approval process.
- Fast-Track Designation – The purpose of a fast-track designation is for the FDA to help researchers develop new treatments, review the safety and effectiveness data, and get new drugs to people as quickly as possible. A drug that receives this designation gets close collaboration with the FDA throughout the testing process to ensure that any problems are caught early and resolved, resulting in earlier drug approvals.
FDA Approval Types
Once researchers have conducted the appropriate testing and research, they may submit an application to gain FDA approval. The FDA will review the data provided and then decide whether to approve the drug for sale in the U.S.
- New Drug Approval – Researchers compile drug information and file a new drug application. This is a comprehensive report that includes all the data and analysis from initial laboratory testing through phase 3 clinical trial results. The application also includes safety data, proposed labeling, and directions for use. Once the application is submitted, the FDA has 6 to 10 months to review the information and decide whether to approve the drug or not. When the FDA is confident the drug is safe and effective, they will approve the drug for patient use. Approved drugs often continue being tested in large populations with phase 4 clinical trials.
- Accelerated Approval – An accelerated approval is a temporary approval by the FDA that is based on a less rigorous endpoint. For example, a cancer drug might receive accelerated approval based largely on safety data and radiographic images showing tumor shrinkage. This designation would allow the drug to be sold on the market and people who need it would gain access to the drug quickly. However, clinical trial testing of the drug would continue and if the trial demonstrates a benefit to people, the drug could receive regular (or full) FDA approval. On the other hand, if the trial doesn’t show a benefit, the drug could be removed from the market.
- Label Expansions – Once a drug has been approved to treat a condition, researchers often consider additional uses for the drug. When researchers can demonstrate a drug is safe and effective for treating an additional disease, they can apply for a label expansion. After reviewing the data, the FDA can approve a label expansion, which allows the drug to be prescribed to treat the additional condition.
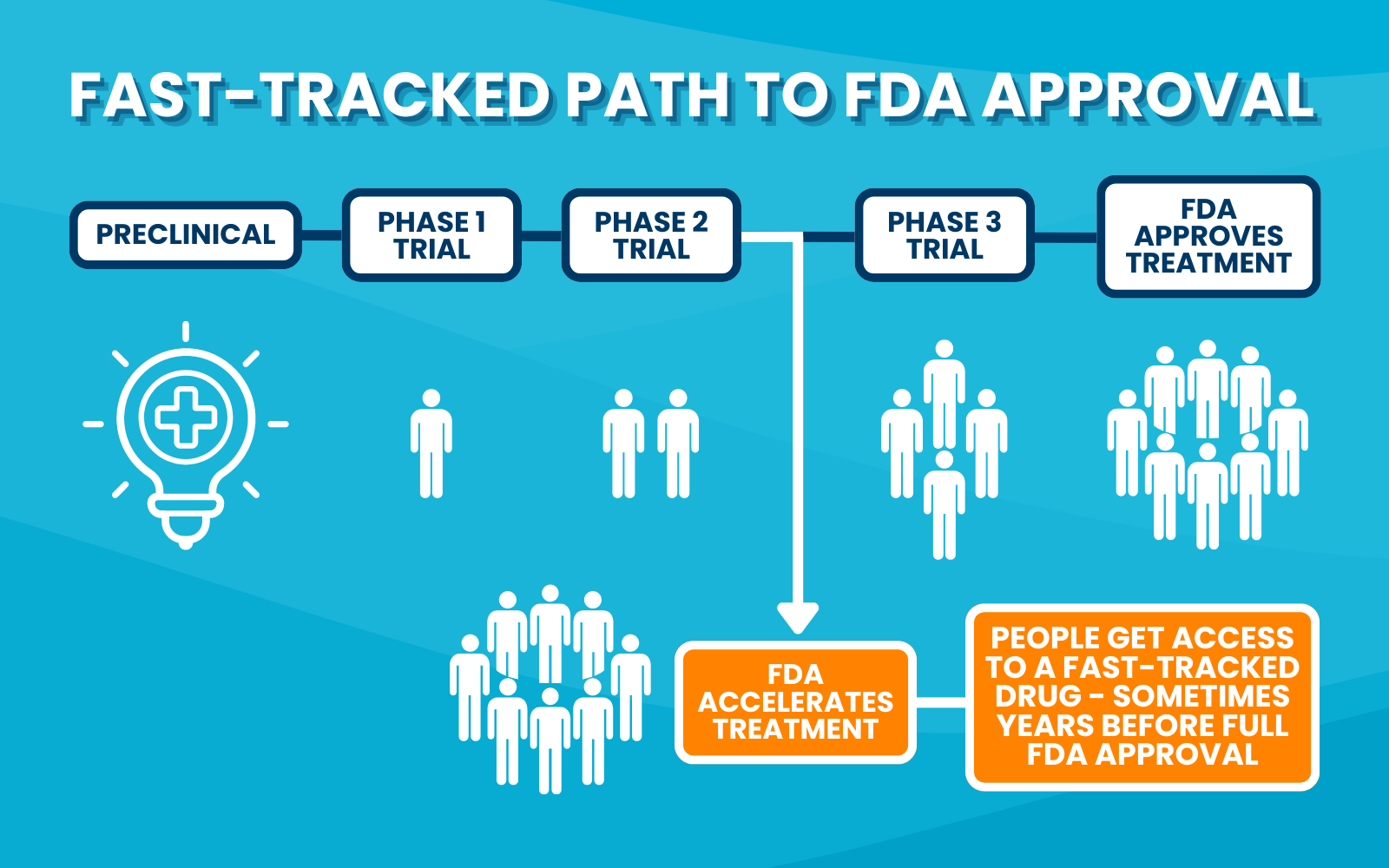
As we’ve described, clinical trials, updated endpoints, and accelerated approval processes are all ways in which researchers and the FDA work together to quickly bring the safest and most effective treatments to people living with lung cancer.
Thank you for joining LUNGevity for our three-part series on how the FDA approval process works. If you like this type of information and want more of it, make sure to sign up for our research email list.

